A BRIEF HISTORY OF BLOOD component EXCHANGE
Blood Exchange Today
The relatively recent spate of publicity around scientific discovery of cellular rejuvenation resulting from exchanging the blood of young mice and old mice focused attention on implications for humans. In an ironic twist of historical and scientific fate, immunologists have exchanged blood and blood components in humans for many decades: every day, clinics, hospitals, and research institutes around the world exchange blood and blood components such as plasma, platelets, red blood cells, and white blood cells, performing approximately 1.2 million procedures per year. Collectively known as apheresis, immunologists use a variety of blood cell separation, collection, and transfusion therapies to treat a range of autoimmune disorders such as multiple sclerosis and Rheumatoid arthritis (RA), certain cancers such as leukemia, specific chronic inflammatory conditions like chronic inflammatory demyelinating polyneuropathy (CIDP), and a host of other indications. More recently, immunologists have begun to use Therapeutic Plasma Exchange, a specific type of apheresis, to address age-related disorders such as Alzheimer's disease.
The relatively recent spate of publicity around scientific discovery of cellular rejuvenation resulting from exchanging the blood of young mice and old mice focused attention on implications for humans. In an ironic twist of historical and scientific fate, immunologists have exchanged blood and blood components in humans for many decades: every day, clinics, hospitals, and research institutes around the world exchange blood and blood components such as plasma, platelets, red blood cells, and white blood cells, performing approximately 1.2 million procedures per year. Collectively known as apheresis, immunologists use a variety of blood cell separation, collection, and transfusion therapies to treat a range of autoimmune disorders such as multiple sclerosis and Rheumatoid arthritis (RA), certain cancers such as leukemia, specific chronic inflammatory conditions like chronic inflammatory demyelinating polyneuropathy (CIDP), and a host of other indications. More recently, immunologists have begun to use Therapeutic Plasma Exchange, a specific type of apheresis, to address age-related disorders such as Alzheimer's disease.
 IBM 2997 Blood Cell Separator circa 1970's
IBM 2997 Blood Cell Separator circa 1970's
Modern History of Blood Exchange: WWII to present
Manual methods of blood component separation and "plasma fractionation" began in earnest over 75 years ago during World War II: Dr. Edwin Cohn, of Harvard University, led efforts to harvest albumin from plasma to help soldiers suffering from shock and burns, maintain adequate intravascular volume during large fluid shifts associated with these conditions. In 1951, working with Dr. Cohn, Dr. José Antonio Grifols Lucas, of
Laboratorios Grifols in Spain, developed the process of plasmapheresis, a method of obtaining plasma that is now used worldwide, and a critical precursor to automated plasmapheresis. Modern therapeutic apheresis began in the 70's when the IBM Corporation developed and commercialized the 2997 Blood Cell Separator, which automated extraction of red blood cells, white blood cells, platelets, or plasma by using centrifugal force to separate blood components by specific gravity and then continuously flow diverting user specified components into a novel collection chamber. Invented by George Judson, an IBM engineer whose son Tom had been diagnosed with Leukemia, George originally worked with the U.S. National Cancer Institute in the 60's to develop the blood cell separation technology. After personally observing cumbersome, time-intensive manual methods to extract white blood cells from leukemia patients such as his son, as well as harvest replacement white blood cells from blood donors, he invented the continuous centrifuge device which gave birth to a new era of automated blood component exchange as well as collection: donors could donate platelets, plasma, or white blood cells weekly, for example, instead of whole blood every two months which then had to be further processed; blood banks and hospitals could more efficiently supply just the needed components to patients.
Manual methods of blood component separation and "plasma fractionation" began in earnest over 75 years ago during World War II: Dr. Edwin Cohn, of Harvard University, led efforts to harvest albumin from plasma to help soldiers suffering from shock and burns, maintain adequate intravascular volume during large fluid shifts associated with these conditions. In 1951, working with Dr. Cohn, Dr. José Antonio Grifols Lucas, of
Laboratorios Grifols in Spain, developed the process of plasmapheresis, a method of obtaining plasma that is now used worldwide, and a critical precursor to automated plasmapheresis. Modern therapeutic apheresis began in the 70's when the IBM Corporation developed and commercialized the 2997 Blood Cell Separator, which automated extraction of red blood cells, white blood cells, platelets, or plasma by using centrifugal force to separate blood components by specific gravity and then continuously flow diverting user specified components into a novel collection chamber. Invented by George Judson, an IBM engineer whose son Tom had been diagnosed with Leukemia, George originally worked with the U.S. National Cancer Institute in the 60's to develop the blood cell separation technology. After personally observing cumbersome, time-intensive manual methods to extract white blood cells from leukemia patients such as his son, as well as harvest replacement white blood cells from blood donors, he invented the continuous centrifuge device which gave birth to a new era of automated blood component exchange as well as collection: donors could donate platelets, plasma, or white blood cells weekly, for example, instead of whole blood every two months which then had to be further processed; blood banks and hospitals could more efficiently supply just the needed components to patients.
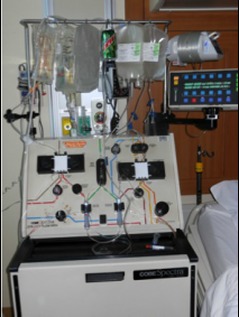 COBE Spectra blood cell separator
COBE Spectra blood cell separator
In 1984, IBM sold its Biomedical Systems business unit to COBE Laboratories, and the redesigned and renamed COBE Spectra blood cell separator became the mainstay of the apheresis industry for the many decades since.
Therapeutic Plasma Exchange - the predominant use of apheresis
Derived from the Greek word aphaíresis – meaning "to take away" – the most prevalent form of apheresis, Therapeutic Plasma Exchange (TPE), intravenously removes a patient’s plasma while simultaneously replacing it with donor plasma or plasma components, such as albumin, known to posses powerful anti-oxidant properties, or plasma derived immunoglobulin's (IVIG), antibodies that the body's immune system uses to identify and neutralize pathogens like bacteria and viruses.
Therapeutic Plasma Exchange - the predominant use of apheresis
Derived from the Greek word aphaíresis – meaning "to take away" – the most prevalent form of apheresis, Therapeutic Plasma Exchange (TPE), intravenously removes a patient’s plasma while simultaneously replacing it with donor plasma or plasma components, such as albumin, known to posses powerful anti-oxidant properties, or plasma derived immunoglobulin's (IVIG), antibodies that the body's immune system uses to identify and neutralize pathogens like bacteria and viruses.
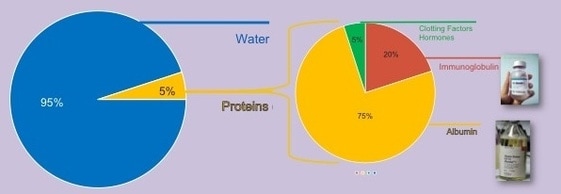 Plasma: ~95% water, 5% proteins
Plasma: ~95% water, 5% proteins
Companies such as Baxter International (U.S.), Grifols S.A. (Spain), Octapharma AG (Switzerland), and CSL Ltd. (Australia), as well as non-profit blood centers such as the Red Cross and Blood Systems (BSI), all use blood cell separators to collect plasma donations, statistically from younger donors. They then sanitize and distill donor plasma, since water makes up approximately 95% of plasma, to extract primary therapeutic plasma proteins such as albumin and imunoglobins for distribution as pharmaceutical grade plasma products available worldwide. Albumin is a protein naturally synthesized by our liver on a daily basis. Replacing plasma with Albumin cleans the blood. Albumin also acts as a carrier protein for many substances around the body that are protein bound, and it helps maintain the correct amount of intravascular volume circulating in the body. Immunoglobulins (antibodies) function as a as critical actors in the humoral immune system, helping the body fight a variety of infections and can be used to support people who have conditions where their immune system is having difficulty producing antibodies (immunodeficiency). Using modern, automated plasma fractionation systems, plasma protein therapeutics manufacturers, complying with global safety regulations, process donated plasma using a variety of purification methods such as solvent/detergent treatment, pasteurization (heating), low pH exposure, and ultraviolet rays, to inactivate and remove viruses, ensuring product safety and efficacy.

Immunologists use IVIG to replace immunoglobulins following plasmapheresis, and transfusion medicine utilizes donated blood components for a wide variety of apheresis procedures. Today, Therapeutic Plasma Exchange (TPE) ranks as the #1 apheresis therapy, largely prescribed for indications of autoimmune disorders, with about 200,000 plasma exchange procedures performed worldwide each year.
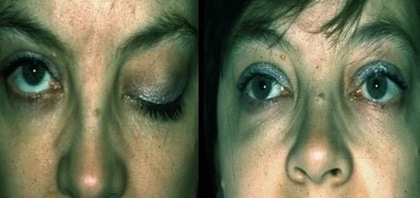 Myasthenia Gravis:
Before and After plasmapheresis
Myasthenia Gravis:
Before and After plasmapheresis
Immunologists have used plasmapheresis for many decades to treat numerous autoimmune disorders such as Rheumatoid arthritis, Lupus, Inflammatory bowel disease (IBD), Multiple sclerosis (MS), Type 1 diabetes mellitus, Psoriasis, Graves' disease, Hashimoto's thyroiditis, Myasthenia gravis, and Vasculitides. Immunologists sometimes see dramatic results as patients will, in some cases such as Myasthenia Gravis or AIDP, recover from acute crisis conditions during a TPE procedure as the apheresis technology removes old, malfunctioning plasma containing autoantibodies, relieving their condition.
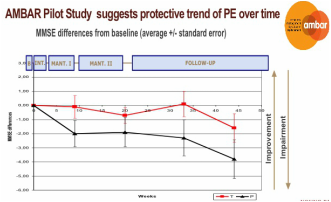 2009 TPE study for Alzheimer's
2009 TPE study for Alzheimer's
Alzheimer's & Aging: Expanding Indications for Therapeutic Plasma Exchange
Historically limited to autoimmune disorders and other less common conditions, recently the use of TPE has expanded as the American Society for Apheresis (ASFA) has published updated evidenced based guidelines for Therapeutic Apheresis to help clinicians. In concert, the US government has expanded the list of billable insurance covered apheresis indications. However, no one previously has undertaken the systemic study of the immunomodulatory benefits of TPE for the broad class of age-associated disorders, which medical data suggests share deficiencies similar to autoimmune disorders.
As more data emerges and clinical trials are performed, some newer, never before considered age-related conditions like Alzheimer’s disease, a disease unique to older people, indicate tremendous promise. For example, a 2009 study of plasma exchange for Alzheimer's patients demonstrated that TPE with 5% Albumin replacement fluid stabilized or improved the condition of patients with mild to moderate Alzheimer’s. A larger follow-up study AMBAR (Alzheimer Management By Albumin Replacement), currently underway for the last two years, has thus far further replicated and confirmed those findings with data to date, prompting, continuation of the study. Notably, these studies, conforming to current medical convention, narrowly measure changes in the specific case of Alzheimer's, but have not measured effects on any other age-related attributes, conditions, or diseases which may exist concurrently as a result of system decline in immune system functionality over time. Grifols recently presented summary findings of their AMBAR study, including high-level results (phase IIb/III) at the Clinical Trials on Alzheimer's Disease (CTAD) congress on October 27, 2018 in Barcelona. Over the course of a 14 month plasma exchange protocol using 5% Albumin and IVIG, Grifols announced that, with statistical significance, the plasma exchange treatment therapy demonstrated a significant reduction (61%) in slowing the progression of the disease in moderate AD patients.
And until relatively recent work with mice in the scientific community, medical practitioners had simply not fully considered or scientifically measured the broader effects of aging blood on age-associated disorders, and correlations between the age of blood, immune system function, and stem cell behavior.
Historically limited to autoimmune disorders and other less common conditions, recently the use of TPE has expanded as the American Society for Apheresis (ASFA) has published updated evidenced based guidelines for Therapeutic Apheresis to help clinicians. In concert, the US government has expanded the list of billable insurance covered apheresis indications. However, no one previously has undertaken the systemic study of the immunomodulatory benefits of TPE for the broad class of age-associated disorders, which medical data suggests share deficiencies similar to autoimmune disorders.
As more data emerges and clinical trials are performed, some newer, never before considered age-related conditions like Alzheimer’s disease, a disease unique to older people, indicate tremendous promise. For example, a 2009 study of plasma exchange for Alzheimer's patients demonstrated that TPE with 5% Albumin replacement fluid stabilized or improved the condition of patients with mild to moderate Alzheimer’s. A larger follow-up study AMBAR (Alzheimer Management By Albumin Replacement), currently underway for the last two years, has thus far further replicated and confirmed those findings with data to date, prompting, continuation of the study. Notably, these studies, conforming to current medical convention, narrowly measure changes in the specific case of Alzheimer's, but have not measured effects on any other age-related attributes, conditions, or diseases which may exist concurrently as a result of system decline in immune system functionality over time. Grifols recently presented summary findings of their AMBAR study, including high-level results (phase IIb/III) at the Clinical Trials on Alzheimer's Disease (CTAD) congress on October 27, 2018 in Barcelona. Over the course of a 14 month plasma exchange protocol using 5% Albumin and IVIG, Grifols announced that, with statistical significance, the plasma exchange treatment therapy demonstrated a significant reduction (61%) in slowing the progression of the disease in moderate AD patients.
And until relatively recent work with mice in the scientific community, medical practitioners had simply not fully considered or scientifically measured the broader effects of aging blood on age-associated disorders, and correlations between the age of blood, immune system function, and stem cell behavior.
2005-2016: Universities measure systemic effects of aging blood & STEM CELL rejuvenation
The "young blood" discovery
In 2005, Michael and Irina Conboy, PhD research scientists then working at Stanford University, resurrected a centuries-old research technique called heterochronic parabiosis (which conjoins two mice of different ages) in a new way. In a landmark study, they measured the ability of stem cells to sustain or regenerate tissues, functionality which declines in all mammals over time, in order to determine whether stem cell functionality deteriorates irreversibly with time; or whether the environment in which the stem cells reside, the blood or "systemic millieu," govern stem cell behavior. Remarkably, the study results uniquivically concluded that the age of the stem cell environment or systemic millieu (plasma) contributed significantly to regenerative potential of stem cells in older individuals (mice). Using genetic lineage tracing (a technique to identify all progeny of a single cell), they verified that tissue regeneration occurred within stem cells already resident in the older mouse and not stem cells which might have migrated from the young mouse to the older mouse within their shared circulatory system. Repetition of the same study protocol by other scientists at UC San Francisco, et al. reaffirmed these results. Aging magazine captures much of the history of these efforts in its article Systemic Problems: A perspective on stem cell aging and rejuvenation.
However, in spite of highly sophisticated, accurate measurement technology, heterochronic parabiosis itself, which creates co-circulatory system between the two conjoined mice, lacks precise control in the exchange of blood, obfuscating more exact scientific understanding of cause and effect between relative ratios and predominant influence of young and old blood, until very recently.
In 2005, Michael and Irina Conboy, PhD research scientists then working at Stanford University, resurrected a centuries-old research technique called heterochronic parabiosis (which conjoins two mice of different ages) in a new way. In a landmark study, they measured the ability of stem cells to sustain or regenerate tissues, functionality which declines in all mammals over time, in order to determine whether stem cell functionality deteriorates irreversibly with time; or whether the environment in which the stem cells reside, the blood or "systemic millieu," govern stem cell behavior. Remarkably, the study results uniquivically concluded that the age of the stem cell environment or systemic millieu (plasma) contributed significantly to regenerative potential of stem cells in older individuals (mice). Using genetic lineage tracing (a technique to identify all progeny of a single cell), they verified that tissue regeneration occurred within stem cells already resident in the older mouse and not stem cells which might have migrated from the young mouse to the older mouse within their shared circulatory system. Repetition of the same study protocol by other scientists at UC San Francisco, et al. reaffirmed these results. Aging magazine captures much of the history of these efforts in its article Systemic Problems: A perspective on stem cell aging and rejuvenation.
However, in spite of highly sophisticated, accurate measurement technology, heterochronic parabiosis itself, which creates co-circulatory system between the two conjoined mice, lacks precise control in the exchange of blood, obfuscating more exact scientific understanding of cause and effect between relative ratios and predominant influence of young and old blood, until very recently.
 Blood exchange with mice
Blood exchange with mice
Revisiting "young blood" in 2016 findings
In another first, for a new 2016 study at UC Berkeley, the Conboys developed a technology more closely resembling human blood cell separators in order to more precisely exchange blood between mice without joining them, and repeated the experiments of 2005 et al., but without the influence of shared organs or other co-circulatory functions. With the new miniaturized blood exchange technology for mice, researchers could control relative amounts of young and old blood exchanged between young and old mice as well as measure effects of the exchange more precisely. Again, they observed changes within 24 hours of blood exchange on the health and repair of multiple tissues, including muscle, liver and brain. But notably, older mice that received younger blood saw either slight or no significant improvements, while young mice that received older blood, saw large declines in most of these tissues or organs. Further, older mice showed no specific improvement in brain neuron stem cells after receiving younger blood, but younger mice that received older blood saw a more than twofold drop in brain cell development compared to normal young mice. In short, old blood appears to have inhibitors of brain cell health and growth, preventing or limiting stem cell functionality.
Another university researcher, Dr. Toney Weiss-Corey of Stanford University, has taken a different research course: first, in 2014, the Stanford team injected the blood of young mice into old mice with young blood His team injected blood plasma from young mice into old mice and showed an improvement in the old mice’s physical endurance and cognitive function. In a follow-on study, his team collected blood samples from 18-year-old human blood donors, and injected them into 12-month-old mice (equivalent to a 50 year old human). After three weeks of twice-weekly injections, the treated mice’s performance (compared to young, 3-month-old mice, and old mice who had not received injections) improved in cognition and memory. As in prior historical experiments in mice, the treated mice appeared to experience neurogenesis, the growth of new brain cells.
Humans are not mice
In any case, scientific studies under controlled conditions with homogenenous subjects (mice) do not necessarily inform practical applications for human medicine. And crude blood and organ sharing via heterochronic parabiosis or whole blood exchange with one-off prototype devices do not remotely compare with the sophisticated evolution of human blood cell separation, donation, and blood component exchange occuring daily in modern medicine. But these experiments do highilight the bioscientific insights possible with highly sophisticated instrumentation, and particularly mass spectrometry (MS), now a virtually ubiquitous research tool, which has illuminated the discovery of isotopes, atomic weights, the characterization of new elements, rapid identification of trace pollutants and drugs, characterization of molecular structure, and now, blood science.
In another first, for a new 2016 study at UC Berkeley, the Conboys developed a technology more closely resembling human blood cell separators in order to more precisely exchange blood between mice without joining them, and repeated the experiments of 2005 et al., but without the influence of shared organs or other co-circulatory functions. With the new miniaturized blood exchange technology for mice, researchers could control relative amounts of young and old blood exchanged between young and old mice as well as measure effects of the exchange more precisely. Again, they observed changes within 24 hours of blood exchange on the health and repair of multiple tissues, including muscle, liver and brain. But notably, older mice that received younger blood saw either slight or no significant improvements, while young mice that received older blood, saw large declines in most of these tissues or organs. Further, older mice showed no specific improvement in brain neuron stem cells after receiving younger blood, but younger mice that received older blood saw a more than twofold drop in brain cell development compared to normal young mice. In short, old blood appears to have inhibitors of brain cell health and growth, preventing or limiting stem cell functionality.
Another university researcher, Dr. Toney Weiss-Corey of Stanford University, has taken a different research course: first, in 2014, the Stanford team injected the blood of young mice into old mice with young blood His team injected blood plasma from young mice into old mice and showed an improvement in the old mice’s physical endurance and cognitive function. In a follow-on study, his team collected blood samples from 18-year-old human blood donors, and injected them into 12-month-old mice (equivalent to a 50 year old human). After three weeks of twice-weekly injections, the treated mice’s performance (compared to young, 3-month-old mice, and old mice who had not received injections) improved in cognition and memory. As in prior historical experiments in mice, the treated mice appeared to experience neurogenesis, the growth of new brain cells.
Humans are not mice
In any case, scientific studies under controlled conditions with homogenenous subjects (mice) do not necessarily inform practical applications for human medicine. And crude blood and organ sharing via heterochronic parabiosis or whole blood exchange with one-off prototype devices do not remotely compare with the sophisticated evolution of human blood cell separation, donation, and blood component exchange occuring daily in modern medicine. But these experiments do highilight the bioscientific insights possible with highly sophisticated instrumentation, and particularly mass spectrometry (MS), now a virtually ubiquitous research tool, which has illuminated the discovery of isotopes, atomic weights, the characterization of new elements, rapid identification of trace pollutants and drugs, characterization of molecular structure, and now, blood science.
common findings in MICE and MEN
Though limited to whole blood exchange, the 2016 Berkeley University study in mice comes the closest yet to approximating modern blood exchange practiced in humans for the past 40 years, and the study confirms basic biological responses in mice which comport with many decades of experience and tens of millions of human procedures worldwide: namely, the critical importance of removing inhibitory factors in blood (plasma) typically associated with declining immune systems, a prevalent condition as blood ages.
In the medical community, it is well established that patients with autoimmune and chronic inflammatory conditions require removal of malfunctioning immune system factors in blood plasma. Decades of work by immunologists with specific chronic inflammatory disorders such as chronic inflammatory demyelinating polyneuropathy (CIDP), a chronic inflammatory disease of the peripheral nervous system (PNS), and acute inflammatory demyelinating polyneuritis, the most common presentation of Guillain–Barré syndrome (GBS), demonstrate a dramatic relief of chronic inflammatory conditions as a result of removal or reduction of disease causing plasma.
Many of the immune system's chemical mediators and signaling molecules exist in the plasma, playing a key role in the maintenance of stem cell activity levels. As our blood ages, stem cell activity typically declines, which leads to a further inability of the body to regenerate cells necessary to recover from afflictions.
In the medical community, it is well established that patients with autoimmune and chronic inflammatory conditions require removal of malfunctioning immune system factors in blood plasma. Decades of work by immunologists with specific chronic inflammatory disorders such as chronic inflammatory demyelinating polyneuropathy (CIDP), a chronic inflammatory disease of the peripheral nervous system (PNS), and acute inflammatory demyelinating polyneuritis, the most common presentation of Guillain–Barré syndrome (GBS), demonstrate a dramatic relief of chronic inflammatory conditions as a result of removal or reduction of disease causing plasma.
Many of the immune system's chemical mediators and signaling molecules exist in the plasma, playing a key role in the maintenance of stem cell activity levels. As our blood ages, stem cell activity typically declines, which leads to a further inability of the body to regenerate cells necessary to recover from afflictions.
AGed blood profile similar to chronic inflammatory and autoimmune disorders
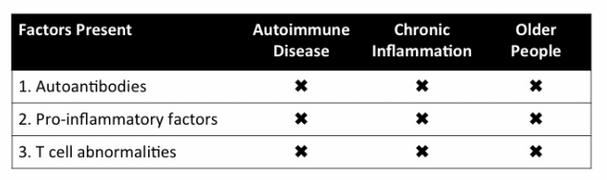 3 Factors commonly present in Aging Blood, Chronic Inflammation, and Autoimmune Disease
3 Factors commonly present in Aging Blood, Chronic Inflammation, and Autoimmune Disease
A declining immune system, whether resultant from an autoimmune disorder, chronic inflammation, and/or aging blood exhibits the presence of autoantibodies, pro-inflammatory factors, and T cell abnormalities. All significant in their ability to cause diseases and aging, these three factors significantly limit the body's ability to repair itself naturally, fight infection, and recover from afflictions.
Autoantibodies. Antibodies form in the body as a response to infection. When an invader (antigen) enters the body, white blood cells known as B lymphocytes react by making special types of proteins called antibodies. Antibodies are your body’s way of remembering an antigen; if it enters the body again, the antibodies will recognize it, combine with it, and neutralize it to prevent you from becoming infected. However, with autoimmune diseases, some of these helpful antibodies can cross react against our own tissues, becoming auto-antibodies that attack your body’s cells as though they were invaders, causing inflammation, damage, and even tissue destruction. A number of studies have shown the presence of autoantibodies in elderly populations.
Pro-inflammatory factors. Chronic (persistent) inflammation is a major underlying condition of many age-related diseases, such as atherosclerosis, arthritis, cancer, diabetes, osteoporosis, dementia, vascular diseases, obesity and metabolic syndrome. According to the CDC, chronic diseases account for 70% of dealths in the U.S. Most chronic inflammatory conditions tend to develop in later stages of life as the immune system declines. A 2015 study entitled "Inflammation, But Not Telomere Length, Predicts Successful Ageing at Extreme Old Age: A Longitudinal Study of Semi-supercentenarians" concluded that lack of inflammation predicted extraordinary lifespan more than any other factor, including telomere length. Fortunately, we can also test blood for presence of pro-inflammatory factors such as Fibrinogen, IL 6, TNF alpha, and C-reactive protein (CRP). For example, CRP, an acute-phase protein increases (or decreases) by 25% or more during inflammatory disorders can rise as high as 1000-fold with inflammation. Fibrinogen (and anticardiolipin antibodies), enhance blood clotting, increasing the likelihood of heart attack and stroke in older patients.
T cell abnormalities. T cells, also known as T lymphocytes help the body's immune system at the cellular level. Cell-mediated immunity fights and removes virus-infected cells, as well as help the body defend against fungi, protozoans, cancers, and intracellular bacteria. Produced or processed by the thymus gland, T cells actively participate in the body's immune response, and number of different immunologic defects have been attributed to T-cell abnormalities in and immunodeficiency.
Indeed, aging of the immune system (“immunosenescence”) affects every attribute we associate with age from chronic diseases to wrinkled skin. According to Dr. Janko Nikolich-Žugich, head of the Department of Immunobiology and Elizabeth Bowman Professor of Medical Research at the UA College of Medicine -- Tucson, "Older adults are by far the largest group of people who are vulnerable to infections, because of their weakened immune systems."
Autoantibodies. Antibodies form in the body as a response to infection. When an invader (antigen) enters the body, white blood cells known as B lymphocytes react by making special types of proteins called antibodies. Antibodies are your body’s way of remembering an antigen; if it enters the body again, the antibodies will recognize it, combine with it, and neutralize it to prevent you from becoming infected. However, with autoimmune diseases, some of these helpful antibodies can cross react against our own tissues, becoming auto-antibodies that attack your body’s cells as though they were invaders, causing inflammation, damage, and even tissue destruction. A number of studies have shown the presence of autoantibodies in elderly populations.
Pro-inflammatory factors. Chronic (persistent) inflammation is a major underlying condition of many age-related diseases, such as atherosclerosis, arthritis, cancer, diabetes, osteoporosis, dementia, vascular diseases, obesity and metabolic syndrome. According to the CDC, chronic diseases account for 70% of dealths in the U.S. Most chronic inflammatory conditions tend to develop in later stages of life as the immune system declines. A 2015 study entitled "Inflammation, But Not Telomere Length, Predicts Successful Ageing at Extreme Old Age: A Longitudinal Study of Semi-supercentenarians" concluded that lack of inflammation predicted extraordinary lifespan more than any other factor, including telomere length. Fortunately, we can also test blood for presence of pro-inflammatory factors such as Fibrinogen, IL 6, TNF alpha, and C-reactive protein (CRP). For example, CRP, an acute-phase protein increases (or decreases) by 25% or more during inflammatory disorders can rise as high as 1000-fold with inflammation. Fibrinogen (and anticardiolipin antibodies), enhance blood clotting, increasing the likelihood of heart attack and stroke in older patients.
T cell abnormalities. T cells, also known as T lymphocytes help the body's immune system at the cellular level. Cell-mediated immunity fights and removes virus-infected cells, as well as help the body defend against fungi, protozoans, cancers, and intracellular bacteria. Produced or processed by the thymus gland, T cells actively participate in the body's immune response, and number of different immunologic defects have been attributed to T-cell abnormalities in and immunodeficiency.
Indeed, aging of the immune system (“immunosenescence”) affects every attribute we associate with age from chronic diseases to wrinkled skin. According to Dr. Janko Nikolich-Žugich, head of the Department of Immunobiology and Elizabeth Bowman Professor of Medical Research at the UA College of Medicine -- Tucson, "Older adults are by far the largest group of people who are vulnerable to infections, because of their weakened immune systems."
aging BLOOD loses immune system functionality
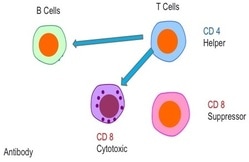
The Immune System. The immune system is comprised of a network of cells, tissues, and organs that work together to defend the body against attacks by “foreign” invaders or pathogens such as microbes — tiny organisms such as bacteria, parasites, and fungi that can cause infections. Most prominent of immune system cells are lymphocytes. Lymphocytes divide into 2 major groups: B cells and T cells. T cells then subdivide into CD4 "helper" cells or CD8 "suppressor" cells, which regulate immune system function.
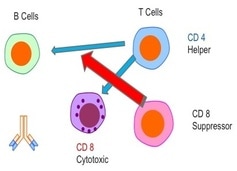
Under normal circumstances, the CD4 helper cells help induce the immune system by sending signals to the B cells -- the "soldiers" of immune system. The B cells have their own antibodies, which destroy viruses, cancer, bacteria, and everything the immune system recognizes as foreign. Once the infection,r virus, or disease is killed, the CD8 cells down-regulate the immune system back to its normal state.
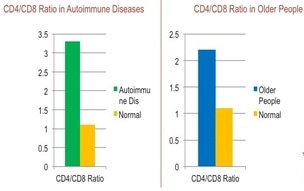 CD4/CD8 ratios in Autoimmune and Aged patients
CD4/CD8 ratios in Autoimmune and Aged patients
The relative ratio of CD4 "helper" cells to CD8 "suppressor" cells changes as blood ages and loses its immune system functionality, a process known as "immunosenescence", and bears close resemblance to the imbalanced ratios of autoimmune diseases than normal ratios. As a marker and predictor of the combined effects of inflammation and immunological changes, the CD4:CD8 ratio has been linked to continuing immune dysfunction, aging, and acts as a predictor of mortality in the general population.
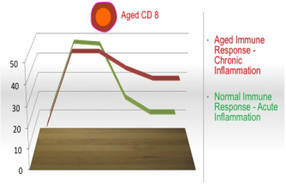 Immune Response to Infection or Injury
Immune Response to Infection or Injury
Chronic Inflammation. The CD4/CD8 ratio, amongst other factors, may represent a bellwether of the combined effects of inflammation and immunological changes called “inflammaging” which, as our blood ages, result in disordered immune responses that do not down-regulate effectively once infection or virus or disease has been neutralized, resulting in chronic (persistent) inflammation. With chronic inflammation, the body operates on high alert in a prolonged state of emergency, which can systemically cause lasting damage to the heart, brain and other organs, which, in turn, increases the incidence of cardiovascular disease, strokes, dementia, cancer, autoimmune conditions, and other physical limitations. As people age, incidence of chronic inflammation increases, thereby increasing the frequency and range of diseases caused by inflammation which cannot be remedied by an overworked and ineffective immune system. In short, aged and ineffective immune systems cause chronic inflammation that, in turn, causes diseases that the now aged and dysfunctional immune system cannot fix itself.
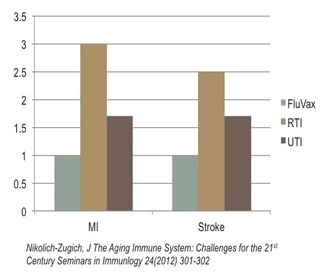 Heart Attack, Stroke Risk in Elderly Post-Infection
Heart Attack, Stroke Risk in Elderly Post-Infection
The systemic effects of chronic inflammation. The risk of myocardial infarction, (MI), (heart attack) after flu vaccine provides a normalized baseline (“1”) to compare against the risk of heart attack in the event of an upper Respiratory Tract Infection (RTI), such as bronchitis or the flu, which results in a 3x greater incidence of myocardial infarction and 2.5 times greater incidence of stroke. Similarly, a Urinary Tract Infection (UTI) results in a 1.6x greater incidence of myocardial infarction, as well as stroke. In other words, the consequence of chronic inflammation increasing with aged immune systems is associated with respiratory or urinary tract infections and can ultimately impact any organ in the body.
Our Blood system: bionetwork for the body
 Blood: the body's network
Blood: the body's network
Our blood system acts as a bionetwork for the body. Blood plasma, 55% of blood volume mainly made up of water, surrounds the red blood cells while transporting key signaling proteins, antibodies, clotting factors, dissolved minerals, hormones and salts, providing the intracorporeal environment within which cells respond. Decades of medical practice evidence the powerful immunomodulatory benefits of plasmapheresis using naturally derived human albumin, immunoglobulins, and other plasma proteins. Though historically limited to autoimmune disorders and other rare conditions, in recent years immunologists have demonstrated common systemic profiles between autoimmune disorders and aging blood, suggesting aging or aging blood is in itself an autoimmune disorder which then leads to age-related diseases such as Alzheimer's, Parkinson's, heart diseases, cancer, and many others. In addition, perhaps more importantly, plasmapheresis may be the world's most powerful, naturally derived immunotherapy to prevent or mitigate age-related conditions.
The last decade's studies in mice have demonstrated equivalent effects, that the biochemical cues transported by blood to and from cells, organs, and tissue within the body uniquely affects every bodily function, regulating and signaling cellular regeneration (youth) or abandonment (aging) in organ stem cells, the body's capacity to repair itself, similar to immune response in humans.
While commonly used in autoimmune disorders, until relatively recently the medical community had simply not fully considered or scientifically measured the potential to rejuvenate stem cells in humans by removing aged plasma and replacing it with young plasma components.
The last decade's studies in mice have demonstrated equivalent effects, that the biochemical cues transported by blood to and from cells, organs, and tissue within the body uniquely affects every bodily function, regulating and signaling cellular regeneration (youth) or abandonment (aging) in organ stem cells, the body's capacity to repair itself, similar to immune response in humans.
While commonly used in autoimmune disorders, until relatively recently the medical community had simply not fully considered or scientifically measured the potential to rejuvenate stem cells in humans by removing aged plasma and replacing it with young plasma components.
